Electron holes on the move
Electron-electron correlation plays a fundamental role in the understanding of the electronic structure of molecules and materials. In systems with strong correlation, a simple description of each electron as moving in an average potential generated by the other electrons fails. In this work, we find evidence for strong correlation between an electron hole and a proton.
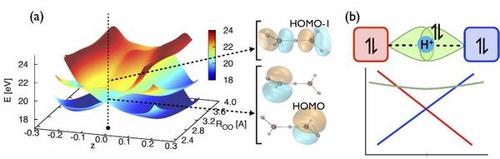
© 2013 American Physical Society
The sudden removal of an electron from a model water cluster leaves behind a positively charged electron hole. In just a few femtoseconds, strong correlation emerges between the hole and the much more massive protons in the system. In soft condensed matter such as water or biological systems, the positively charged protons build bridges, called hydrogen bonds, between heavier atoms such as oxygen or nitrogen. Small displacements of the protons between those atoms can result in relatively large charge reorganization. Here, the sudden presence of the hole triggers proton displacements, to which the hole in turn quickly responds, building up the correlation. The interaction between hole and proton is caused by the Coulomb repulsion between them and results in a complete breakdown of the Born-Oppenheimer approximation, which plays a central role in molecular physics and implies that the lighter electrons instantaneously adapt to the motion of the heavier nuclei.
Our study on proton-hole correlation sets an upper time scale for pure hole dynamics before protons are set in motion by the presence of a hole, and explains the dynamics of water clusters, and possibly of other systems featuring similar building blocks, ionized by free-electron lasers.
Phys. Rev. Lett. 110 (2013) 038302
http://prl.aps.org/abstract/PRL/v110/i3/e038302