A lifetime for ionized acetylene
Photoisomerization, the process by which the absorption of a photon induces geometrical rearrangements of the atoms in a molecule, is an important phenomenon in chemistry and biology. It is, for example, responsible for initiating the cascade of events underlying the process of vision, where photons interact with retinal molecules in the eye.
© 2011 American Physical Society
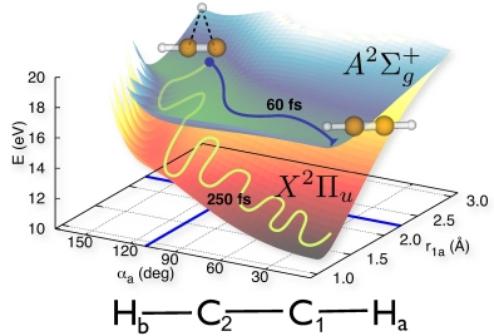
Here, we simulate the isomerization of acetylene after photoionization.Photoionization leaves the acetylene molecule in an electronically excited state. In this electronically excited state, isomerization takes place and a hydrogen atom is transferred from one carbon atom to the other in about 100 fs. At the new geometry resulting from isomerization, the electronic energy stored
in the molecule is transformed into vibrational energy---in a process that violates an assumption widely adopted in molecular physics, which is known as the Born-Oppenheimer approximation. According to our calculations, the energy redistribution into molecular vibrations is completed in less than 500 fs. The vibrationally hot molecule adopts a new quasi-equilibrium geometry similar to
the original structure of neutral acetylene.
The reported results explain recent experiments at the free-electron laser in Hamburg (FLASH), and predict an efficient vibrational energy redistribution mechanism that has not yet been accessed experimentally.
Phys. Rev. Lett. 107 (2011) 263002
http://prl.aps.org/abstract/PRL/v107/i26/e263002